Resonance Assignment/Abacus/Introduction to ABACUS: Difference between revisions
Jump to navigation
Jump to search
Figure 1.2
Figure 1.4
No edit summary |
RyanDoherty (talk | contribs) No edit summary |
||
| (66 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
= | = ABACUS approach. = | ||
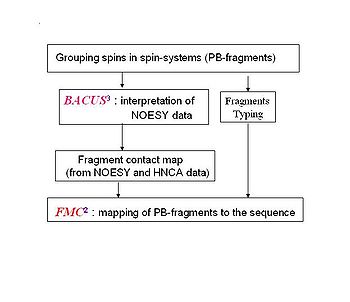
<div>ABACUS (''A''pplied ''BACUS'') is a novel approach for protein structure determination that has been applied successfully for more than 20 NESG targets. ABACUS is characterized by use of BACUS, a procedure for automated probabilistic interpretation of NOESY spectra in terms of unassigned proton chemical shifts based on the known information | <div>ABACUS (''A''pplied ''BACUS'') is a novel approach for protein structure determination that has been applied successfully for more than 20 NESG targets. ABACUS is characterized by use of BACUS, a procedure for automated probabilistic interpretation of NOESY spectra in terms of unassigned proton chemical shifts based on the known information about the "connectivity" between proton resonances. BACUS is used in both the resonance assignment and structure calculation steps. The resonance assignment strategy of ABACUS<span> is what distinguishes it the most from conventional NMR structure determination approaches (see Fig.1.1A). </span><br></div> | ||
==== '''Figure 1.1A''' ==== | |||
[[Image:Abacus.JPG|thumb|left|350px]]<br> | |||
<br> | |||
<br> | |||
<br> | |||
<br> | |||
<br> | <br> | ||
<br>''' | <br>''' ''' | ||
| Flowchart of resonance assignment by ABACUS''. '' | ||
<br> | |||
<br> | |||
<br> | |||
<br> | |||
<br> | |||
==== ==== | |||
| |||
| |||
''' | ==== '''<span>Some features /advantages of the ABACUS protocol:</span>''' ==== | ||
*It does not rely on sequential connectivities from less sensitive experiments (ie. HNCACB) that are indispensable for most traditional sequential assignment procedures; | |||
*Inter-residue sequential connectivities are established mainly from NOE data, which saves time while “troubleshooting” NOE and resonance assignments; | |||
* | *Probabilistic nature of the ABACUS procedure provides a measure of reliability for the assignments, and therefore one can obtain a partial, yet highly reliable assignment (even when the NMR data are sub-optimal) because of knowing where to focus manual intervention<font size="3">;</font> | ||
*Inter-residue sequential connectivities are established mainly from NOE data, which saves time | |||
*Probabilistic nature of the ABACUS procedure provides measure of reliability | |||
*It can make use of partial spin-systems; | *It can make use of partial spin-systems; | ||
*It can efficiently identify manual errors in the input peak lists; | *It can efficiently identify manual errors in the input peak lists; | ||
| Line 19: | Line 45: | ||
<br> | <br> | ||
| | ||
= NMR spectra required for ABACUS | = NMR spectra required for ABACUS = | ||
<div></div><div></div><div></div><div>The spectra typically needed for ABACUS approach are most conveniently separated into 3 groups: NH-rooted, the CH-rooted and the aromatic (also CH-rooted). Table 1 shows the optimal set of NMR spectra. This, of course, is neither an exclusive or exhaustive list. For example, a simultaneous CN-NOESY could be recorded instead of three different ones listed in the table. | <div></div><div></div><div></div><div>The spectra typically needed for the ABACUS approach are most conveniently separated into 3 groups: NH-rooted, the CH-rooted and the aromatic (also CH-rooted). Table 1 shows the optimal set of NMR spectra. This, of course, is neither an exclusive or exhaustive list. For example, a simultaneous CN-NOESY could be recorded instead of three different ones listed in the table. For proteins with very few aromatic residues, collecting only one aromatic spectrum (ie. aromatic NOESY) could be sufficient for the assignment of aromatic resonances. </div><div> </div><div>'''Table 1.''' '''ABACUS optimal set of experiments''' </div><div> </div> | ||
{| class="FCK__ShowTableBorders" cellspacing="0" cellpadding="0" border="0" | {| class="FCK__ShowTableBorders" cellspacing="0" cellpadding="0" border="0" | ||
|- | |- | ||
| Line 58: | Line 84: | ||
|} | |} | ||
<div> </div><div> </div><div>'''<font size="5"></font>'''</div> | <div> </div><div> </div><div>'''<font size="5"></font>'''</div> | ||
= Spin-system identification strategy | |||
<div></div><div></div><div></div><div>The resonance assignment procedure starts | = Spin-system identification strategy = | ||
<div></div><div></div><div></div><div>The resonance assignment procedure starts by grouping resonances into spin systems. Two types of spin-systems will be described in this manual.</div><div></div> | |||
==== PB fragment ==== | |||
<div></div><div>PB (Peptide Bond) fragments consist of correlated resonances from the side chain of residue ''i'' and the NH resonances of residue ''i+1'' (see Figure 1.1B).</div><div> | |||
| |||
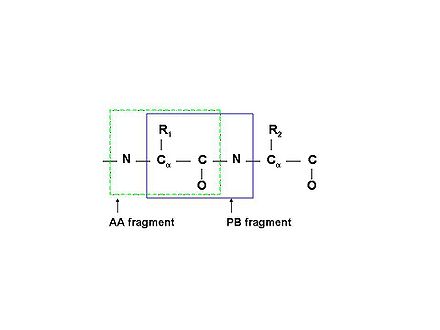
'''Figure 1.1B''' | |||
[[Image:PBfragment.jpg|thumb|right|440px]]Schematic description of two types of molecular fragments: traditional spin-system (AA-fragment)<span> include all the atoms belonging to the same residue; PB-fragment includes all the atoms from one residue except the backbone amide group, plus the amide group from the next residue in the protein</span> | |||
<br> | |||
<br> | |||
<br> | |||
<br> | <br> | ||
| Line 68: | Line 107: | ||
<br> | <br> | ||
= FMC Graphical User Interface | <br> | ||
<div>FMCGUI is a graphical interface that | |||
<br> | |||
<br> | |||
<br> | |||
<br> | |||
</div><div></div> | |||
<br> | |||
<br> | |||
<br> | |||
<br> | |||
==== ''b''PB fragment ==== | |||
<div>Uncompleted HN-rooted PB spin-systems, which include resonances of<span> Cα, Hα, Cβ, and Hβ atoms of residue ''i'' </span>and the NH resonances of residue ''i+1''<span>, are called ''b''PB fragments. </span></div><div> </div><div>Spin-system identification in the ABACUS approach consists of 3 main steps.</div><div> </div><div> 1. During the first step, ''b''PB fragments are collected from high sensitivity NMR correlation experiments (such as HNCO, CBCA(CO)NH, and HBHA(CO)NH ) that transfer magnetization via the intervening peptide bond (see Figure 4.1A). </div><div> </div><div> 2. During the second step, completion of ''b''PB fragments with side-chain aliphatic resonances and identification of additional spin-systems (lacking HN resonances) are performed using HCCH-TOCSY and 13C-NOESY spectra (see Figure 4.1B).</div><div> </div><div> 3. During the final step, spin-system validation and correction are performed. This step allows the user to find mistakes made during spectra peak-picking and to correct the mistakes by referring back to the spectra. </div><div> </div> | |||
<br> | |||
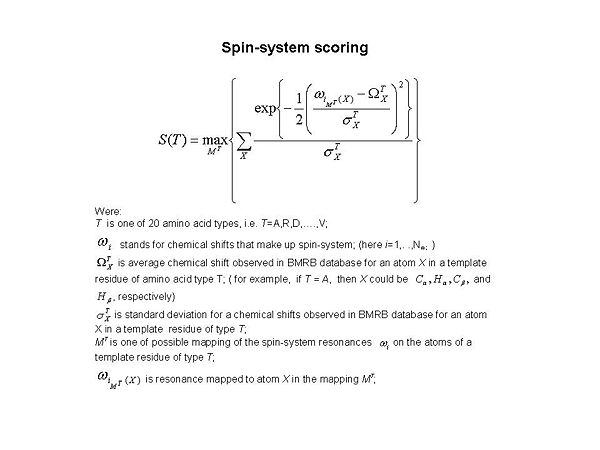
==== '''Figure 1.2<br>''' ==== | |||
<div><span>[[Image:Fmcgui Fig1.2.jpg|thumb|center|600px]] </span></div><div><div>During the validation step, twenty S(T) scores were calculated for each spin-system (see Figure 1.2 ). Here ''T'' corresponds to amino acid type, and ''T ''= A, R, D, …, and V, respectively. The score evaluates goodness-of-fit for the spin-system resonances in comparison to observed data obtained from the BMRB database. When the best S(T) score is low (ie. S<sub>max </sub>< 10<sup>-4</sup>, where <span>S<sub>max </sub></span> = max{ S(T)}), it means that either the spin-system has very unusual chemical shifts or the spin-system does not make sense and needs to be <span>corrected. </span></div><div> </div></div><div><br></div><div>'''<font size="5"></font>'''</div> | |||
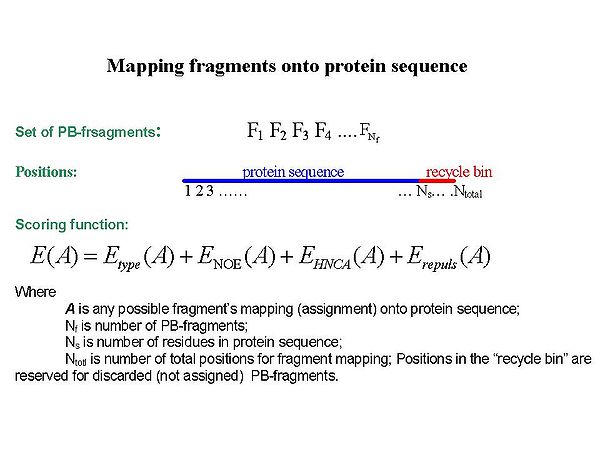
= Fragment assignment by FMC procedure = | |||
<div></div><div></div><div></div><div></div><div>Sequence-specific assignment of PB-fragments is achieved using a Fragment Monte Carlo (FMC) stochastic search procedure. The scoring function used in the FMC procedure is based on both fragment amino acid typing (matching the spin system to amino acid types) and fragment contact map (identifying neighbouring residues) derived from HNCA data and the analysis of NOEs interpreted by BACUS (see Figure 1.3)</div><div> </div> | |||
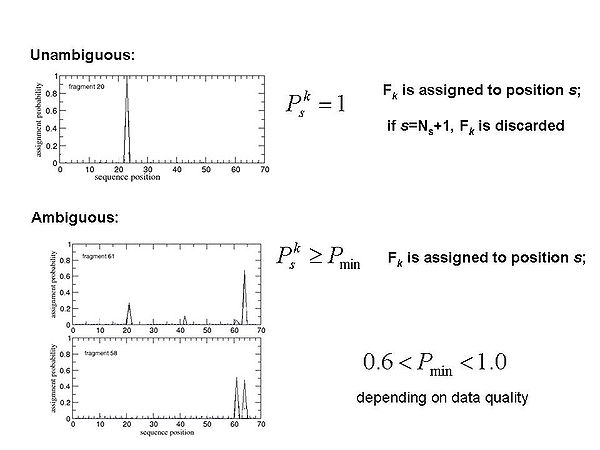
==== Figure 1.3 ==== | |||
<div>[[Image:Fmcgui Fig1.3.jpg|thumb|center|600px]]</div><div></div><div></div><div> FMC procedure performs ''<u>probabilistic assignment</u>'' of PB-fragments. The assignment probabilities <span>P<sub>s</sub><sup>k</sup></span><span> are calculated by Simulated Annealing (SA) or Replica Exchange Method (REM) Monte Carlo (MC) simulations. Here, P<sub>s</sub><sup>k</sup> is a </span>probability of fragment ''k'' to occupy position ''s;'<span id="1259188877701S" style="display: none"> </span>k = 1,….,N<sub>f. ;</sub>''</div> | |||
==== Figure 1.4<br> ==== | |||
[[Image:Fmcgui Fig1.4.jpg|thumb|center|600px]] | |||
= FMC Graphical User Interface = | |||
<div>FMCGUI is a graphical interface that allows the user to carry out resonance assignment and structure calculation using the ABACUS approach. FMCGUI integrates a number of FORTRAN applications: controlling the data-flow between the applications, executing applications, and visualizing data for effective analysis of results. <br></div><div></div><div>The main purpose of FMCGUI is to provide an interactive tool for resonance assignment. FMCGUI includes a structure calculation component, which can be used independently from the resonance assignment component. The structure calculation component assists the user by setting up structure calculations with CYANA, water refinement calculations with CNS, and performs an analysis of their results. The structure calculations are to be run externally from FMCGUI, on a linux cluster. </div> | |||
Latest revision as of 21:39, 6 January 2010
ABACUS approach.
ABACUS (Applied BACUS) is a novel approach for protein structure determination that has been applied successfully for more than 20 NESG targets. ABACUS is characterized by use of BACUS, a procedure for automated probabilistic interpretation of NOESY spectra in terms of unassigned proton chemical shifts based on the known information about the "connectivity" between proton resonances. BACUS is used in both the resonance assignment and structure calculation steps. The resonance assignment strategy of ABACUS is what distinguishes it the most from conventional NMR structure determination approaches (see Fig.1.1A).
Figure 1.1A
Flowchart of resonance assignment by ABACUS.
Some features /advantages of the ABACUS protocol:
- It does not rely on sequential connectivities from less sensitive experiments (ie. HNCACB) that are indispensable for most traditional sequential assignment procedures;
- Inter-residue sequential connectivities are established mainly from NOE data, which saves time while “troubleshooting” NOE and resonance assignments;
- Probabilistic nature of the ABACUS procedure provides a measure of reliability for the assignments, and therefore one can obtain a partial, yet highly reliable assignment (even when the NMR data are sub-optimal) because of knowing where to focus manual intervention;
- It can make use of partial spin-systems;
- It can efficiently identify manual errors in the input peak lists;
NMR spectra required for ABACUS
The spectra typically needed for the ABACUS approach are most conveniently separated into 3 groups: NH-rooted, the CH-rooted and the aromatic (also CH-rooted). Table 1 shows the optimal set of NMR spectra. This, of course, is neither an exclusive or exhaustive list. For example, a simultaneous CN-NOESY could be recorded instead of three different ones listed in the table. For proteins with very few aromatic residues, collecting only one aromatic spectrum (ie. aromatic NOESY) could be sufficient for the assignment of aromatic resonances.
Table 1. ABACUS optimal set of experiments
NH-rooted
|
CH-rooted
|
Aromatic
|
15N-HSQC
|
13C-CT-HSQC
|
13C-HSQC-aro
|
HNCO
|
13C-HSQC
|
H(C)CH-TOCSY-aro
|
HNCA
|
H(C)CH-TOCSY
|
(H)CCH-TOCSY-aro
|
CBCA(CO)NH
|
(H)CCH-TOCSY
|
13C-NOESY-HSQC-aro
|
HBHA(CO)NH
|
13C-NOESY-HSQC
|
|
15N-NOESY-HSQC
|
||
CCCONH-TOCSY (optional)
| ||
H(CCCO)NH-TOCSY (optional)
| ||
Spin-system identification strategy
The resonance assignment procedure starts by grouping resonances into spin systems. Two types of spin-systems will be described in this manual.
PB fragment
PB (Peptide Bond) fragments consist of correlated resonances from the side chain of residue i and the NH resonances of residue i+1 (see Figure 1.1B).
Figure 1.1B
Schematic description of two types of molecular fragments: traditional spin-system (AA-fragment) include all the atoms belonging to the same residue; PB-fragment includes all the atoms from one residue except the backbone amide group, plus the amide group from the next residue in the protein
bPB fragment
Uncompleted HN-rooted PB spin-systems, which include resonances of Cα, Hα, Cβ, and Hβ atoms of residue i and the NH resonances of residue i+1, are called bPB fragments.
Spin-system identification in the ABACUS approach consists of 3 main steps.
1. During the first step, bPB fragments are collected from high sensitivity NMR correlation experiments (such as HNCO, CBCA(CO)NH, and HBHA(CO)NH ) that transfer magnetization via the intervening peptide bond (see Figure 4.1A).
2. During the second step, completion of bPB fragments with side-chain aliphatic resonances and identification of additional spin-systems (lacking HN resonances) are performed using HCCH-TOCSY and 13C-NOESY spectra (see Figure 4.1B).
3. During the final step, spin-system validation and correction are performed. This step allows the user to find mistakes made during spectra peak-picking and to correct the mistakes by referring back to the spectra.
Figure 1.2
During the validation step, twenty S(T) scores were calculated for each spin-system (see Figure 1.2 ). Here T corresponds to amino acid type, and T = A, R, D, …, and V, respectively. The score evaluates goodness-of-fit for the spin-system resonances in comparison to observed data obtained from the BMRB database. When the best S(T) score is low (ie. Smax < 10-4, where Smax = max{ S(T)}), it means that either the spin-system has very unusual chemical shifts or the spin-system does not make sense and needs to be corrected.
Fragment assignment by FMC procedure
Sequence-specific assignment of PB-fragments is achieved using a Fragment Monte Carlo (FMC) stochastic search procedure. The scoring function used in the FMC procedure is based on both fragment amino acid typing (matching the spin system to amino acid types) and fragment contact map (identifying neighbouring residues) derived from HNCA data and the analysis of NOEs interpreted by BACUS (see Figure 1.3)
Figure 1.3
FMC procedure performs probabilistic assignment of PB-fragments. The assignment probabilities Psk are calculated by Simulated Annealing (SA) or Replica Exchange Method (REM) Monte Carlo (MC) simulations. Here, Psk is a probability of fragment k to occupy position s;'k = 1,….,Nf. ;
Figure 1.4
FMC Graphical User Interface
FMCGUI is a graphical interface that allows the user to carry out resonance assignment and structure calculation using the ABACUS approach. FMCGUI integrates a number of FORTRAN applications: controlling the data-flow between the applications, executing applications, and visualizing data for effective analysis of results.
The main purpose of FMCGUI is to provide an interactive tool for resonance assignment. FMCGUI includes a structure calculation component, which can be used independently from the resonance assignment component. The structure calculation component assists the user by setting up structure calculations with CYANA, water refinement calculations with CNS, and performs an analysis of their results. The structure calculations are to be run externally from FMCGUI, on a linux cluster.