Resonance Assignment/Abacus/FMCGUI commands: Difference between revisions
No edit summary |
|||
| (24 intermediate revisions by one other user not shown) | |||
| Line 13: | Line 13: | ||
<div></div><div> :: Save the current state of the project</div><div> </div><div>What is currently in the computer memory is saved in the file PROJECTNAME.prj located in the root directory of the project. </div><div> </div> | <div></div><div> :: Save the current state of the project</div><div> </div><div>What is currently in the computer memory is saved in the file PROJECTNAME.prj located in the root directory of the project. </div><div> </div> | ||
=== '''<span>Poject>Quit</span>''' === | === '''<span>Poject>Quit</span>''' === | ||
<div>:: Save the current state of the project and quit. | <div> </div><div>:: Save the current state of the project and quit.</div><div> </div> | ||
== Data Menu<br> == | == Data Menu<br> == | ||
<div>The DATA section serves to load&save the data (such as protein sequence and peak lists). Since there are different formats of data-files that could be loaded in memory or saved on disk, one can use this section as format converter as well.</div><div> </div> | <div>The DATA section serves to load&save the data (such as protein sequence and peak lists). Since there are different formats of data-files that could be loaded in memory or saved on disk, one can use this section as format converter as well.</div><div> </div> | ||
| Line 239: | Line 240: | ||
=== '''Structure>Constraints>H-bonds>Specify''' === | === '''Structure>Constraints>H-bonds>Specify''' === | ||
<div>''':''': Specify H-bond distance constraints manually.</div><div> </div><div>''Prerequisites'': </div><div> ♦ protein sequence is loaded in memory</div><div> </div><div>[[Image: | <div>''':''': Specify H-bond distance constraints manually.</div><div> </div><div>''Prerequisites'': </div><div> ♦ protein sequence is loaded in memory</div><div> </div><div>[[Image:Fmcgui fig2.16.jpg|thumb|left|100px]]A new window “O/HN Pairs” is opened (see Figure ). </div><div> </div><div> </div><div></div><div> </div><div> <br> </div><div> </div><div> </div><div></div><div> </div><div></div><div>For each H-bond constraint, user have to specify O-HN pair by typing in the ID of residues corresponding to O and HN atoms. Pressing “OK” button will save H-Bond constraints on disk in two formats: files 'hbond.upl' and 'prot_hbond.tbl' for CYANA and CNS calculations, respectively. Both files are saved in the project root project directory.</div><div> <br></div><div> <br> </div><div></div> | ||
=== '''Structure>Constraints>H-Bonds>load''' === | === '''Structure>Constraints>H-Bonds>load''' === | ||
<div>''':''' | <div>'''::''' Load HB-ond distance constraints in CYANA format (upl-file) </div><div> </div><div> </div> | ||
=== '''Structure>Calculate>Cyana''' === | === '''Structure>Calculate>Cyana''' === | ||
<div>''':''' | <div>''':''': Set up new structure calculation run with CAYANA.</div><div> </div><div>'' Prerequisites'': </div><div> ♦ protein sequence is loaded in memory</div><div> ♦ assigned chemical shifts are loaded in memory</div><div> ♦ N15-NOESY, C13-NOESY, and Arom-NOESY peak lists are loaded in memory</div><div> ♦ specified tolerances</div><div> ♦ dihedral angle constraints are created ( i.e. file “belok.aco” is present in the project root directory)</div><div> ♦'' Optional:''</div><div> ♦ HBond distance constraints (file “hbond.upl” is present in the project root directory)</div><div> ♦ file that contains ZN ion ligands (file “zn_ligands” is present in the project root directory)</div><div> </div><div>A new directory under the name that is specified by user (normally “crun#”) will be created inside project root directory PROJECTNAME. This directory contains all files required to start automatic structure calculations with CYANA.</div><div> </div> | ||
=== '''Structure>Calculate>ABCUS''' === | === '''Structure>Calculate>ABCUS''' === | ||
<div>''':''' | <div>''':''': Sset up ABACUS structure calculations</div><div> </div><div> </div> | ||
=== '''Structure>RPF>RP''' === | === '''Structure>RPF>RP''' === | ||
<div>''':''' | <div>''':''': Perform Recall/Precision analysis of structural ensemble.</div><div> </div><div> ''Prerequisites'': </div><div> ♦ protein sequence is loaded in memory</div><div> ♦ assigned shemical shifts are loaded in memory</div><div> ♦ N15-NOESY, C13-NOESY, and Arom-NOESY peak lists are loaded in memory</div><div> ♦ specified tolerances</div><div> ♦ coordinates of structural ensemble in CYANA format (final.pdb) or in CNS format <br> </div><div> </div> | ||
<br> | ''Results:''<br> | ||
<div><span> </span></div><div> </div><div> </div><div> | |||
♦ summary report<br> | |||
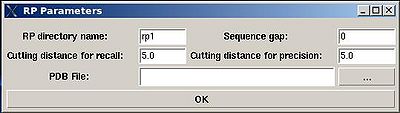
<div> ♦ flase negative and false positive peak lists<span> </span></div><div> </div><div> </div><div> The parameters to set up (see Figure ):</div><div>[[Image:Fmcgui_fig2.17.jpg|thumb|left|400px]] <br></div> | |||
*<span><span> </span></span>“RP directory name”. Normally the name is rp#. A new directory under this name will be created within PROJECTNAME/assign directory. The results of the RPFanalysis will be stored in this directory. | |||
*<span><span> </span></span>“sequence gap”. Residue pairs separated by less than the value of sequence gap will be excluded from generating expected peak lists. | |||
*<span><span> </span></span>“cutting distance for recall”. Distance threshold for evaluating matching of an experimental peak to a structural ensemble (Recall score) | |||
*<span><span> </span></span>“cutting distance for precition”. Distance threshold for generating expected peak from structural ensemble (Precision score) | |||
<div> </div><div>The results of the RPF analysis will be saved in a new directory (for example, directory "rp1" on Figure) that is located in the project root directory. Results include peak lists in the SPARKY format of both false negative and false positive peaks for different NOESY spectra in a separate files.</div><div> </div> | |||
=== '''Structure>RPF>DP''' === | === '''Structure>RPF>DP''' === | ||
:''':''' Set up calculations of DP score with AutoStructure | |||
<div> </div><div>''Prerequisites'': </div><div> | <div> </div><div>''Prerequisites'': </div><div> ♦ protein sequence is loaded in memory</div><div> ♦ assigned shemical shifts are loaded in memory</div><div> ♦ N15-NOESY, C13-NOESY, and Arom-NOESY peak lists are loaded in memory</div><div> ♦ specified tolerances</div><div> ♦ coordinates of structural ensemble in CYANA format (final.pdb) or in CNS format </div><div> </div><div> </div><div> </div> | ||
=== '''Structure>Water refinement>calculate''' === | === '''Structure>Water refinement>calculate''' === | ||
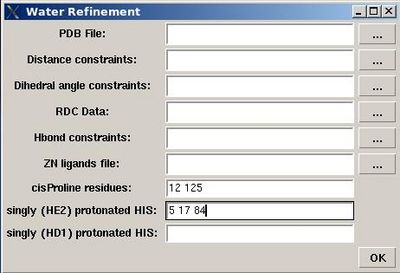
<div> ''':''' | <div> ''':''': Set up water refinement calculations</div><div> </div><div>''Prerequisites'': </div><div> ♦ structure calculation with CYANA should be done</div><div> ♦ ''Optional:''</div><div> ♦ RDC data in PALES format.</div><div> ♦ file with ZN ion ligands (file [[FMCGUI_commands#Structure.3EAdd_ZN_ligands|“zn_ligands”]] )</div><div> <br></div><div> [[Image:Fmcgui fig2.18.jpg|thumb|right|400px]]<br> </div> <div>User will be asked to :</div> | ||
< | * specify the name of directory for water refinement calculations, WATDIR; | ||
<div> | * select a number of files with coordinates and constraints; | ||
* indicate cisProline residues (if there are any) | |||
* specify protonation state of HIS residues (which is double protonated by default). <br> | |||
<div> </div><div> </div><div></div><div>In the result, a new directory WATDIR will be created inside project root directory. I will contain all files and scripts required to carry out water refinement calculations with CNS. </div><div>The calculations should be started outside FMCGUI. (linux cluster is is recommended)</div><div> <br> </div><div> </div><div> <br> </div><div> </div><div> </div><div></div><div></div><div><br></div><div> </div> | |||
=== '''Structure>Water refinement>summary''' === | === '''Structure>Water refinement>summary''' === | ||
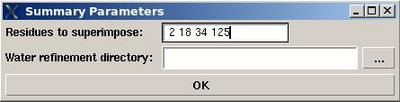
<div>''':''' | <div>'''::''' Create a summary for water refined structural ensemble.</div><div> </div><div>''Prerequisites'': </div><div> ♦ water refinement with CNS should be done.</div><div> </div><div>User will be asked to specify residues used for structure superposition and to select the water refinement directory, WATDIR (see Figure).</div><div> [[Image:Fmcgui_fig2.19.jpg|thumb|right|400px]]</div><div> <span> </span></div><div> </div><div>The refined structural models will be superimpose and combined in one file. The created summary reports values of different energy components and constraint violation statistic for each structural model. A new directory WATDIR_results will be created. The directory contains final superimposed coordinates, distance and dihedral angle constrains in a format suitable for PDB deposition, summary and constraint violations report.</div><div> </div> | ||
=== '''Structure>Add ZN ligands''' === | === '''Structure>Add ZN ligands''' === | ||
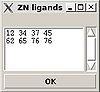
<div>''':''' | <div>''':: '''Create "zn_ligands" file.</div><div> </div><div>[[Image:Fmcgui fig2.20.jpg|thumb|left|100px]]“ZN ligands” window pops up. User have to type in IDs of residues that ligate zinc ion. If there are a few zinc ions, information for each ion should be provided in a separate row (see Figure).</div><div><span> </span></div><div> </div><div> </div><div> </div><div>The file “zn_ligands” will be created inside the project root directory by pressing “OK” button.</div><div> </div> | ||
=== '''Structure>RCI''' === | === '''Structure>RCI''' === | ||
& | ''':''': Calculate Random Coil Index | ||
<div> </div><div> </div><div>Random Coil Index is calculated using rci_v_1c.py script. New directory | <div> </div><div> </div><div>Random Coil Index is calculated using rci_v_1c.py script. New directory "rci", that contains results of the calculations, is created </div><div>inside project root directory.</div><div> </div><div> </div> | ||
== View menu == | == View menu == | ||
<div>This section provides means to visualize data and calculation results.</div><div> </div> | <div>This section provides means to visualize data and calculation results.</div><div> </div> | ||
=== '''View>Fragment''' === | === '''View>Fragment''' === | ||
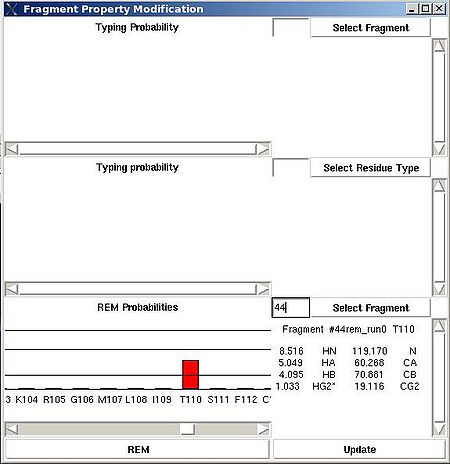
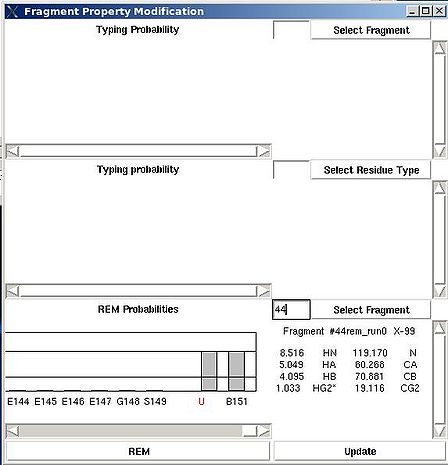
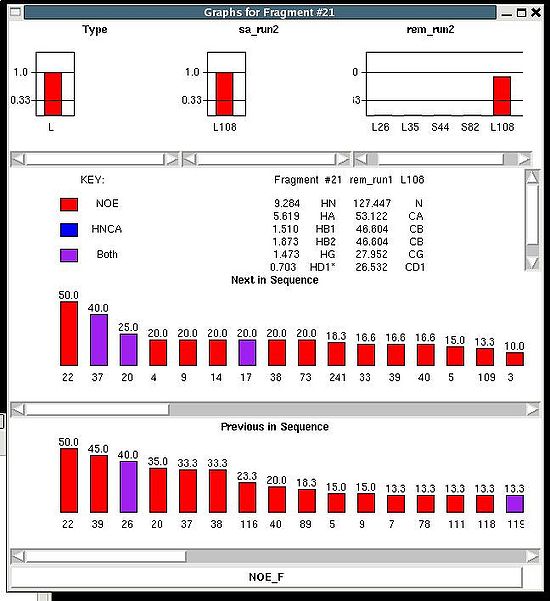
<div> ''':''' | <div> ''':''': Display current properties of selected fragment<br></div><div> </div><div> This command opens “Fragment Graph” (FG) window were all properties of a selected fragment that currently are loaded in memory will be displayed (see Figure). </div><div> [[Image:Fmcgui 2.21.jpg|thumb|left|550px]]</div><div>The chemical shifts making up the fragment are shown in the middle part of the window. The heading line (for example, line “Fragment #21 rem_run1 L108” shown on Figure 2.21) shows fragment ID (#21), the name of directory that contains assignment probabilities used to assign the fragment (rem_run1) and sequence position to which the fragment is assigned (L108). All other fragment properties are shown in the form of graphs. Typing probabilities the top section of the window one can see typing probabilities <span> and both assignment probabilities and are shown on three graph on the top part of the FG window. It is also indicated the name of the directories from which the displayed assignment probabilities were loaded (for example, on the Figure, the P<sub>SA</sub> and P<sub>REM</sub> assignmet probabilities were loaded from directories "sa_run2" and "rem_run2", respectively). Two graphs on the bottom part of the FG window shows a column ( C<sup>f</sup><sub>NOE_B</sub>(''U_id'') or C<sup>f</sup><sub>NOE_F</sub>(''U_id'') ) and a row (C<sup>''U_id''</sup><sub>NOE_B</sub>(f) or </span><span>C<sup>''U_id''</sup><sub>NOE_F</sub>(f) </span><span> ) of contact map calculated from NOESY data, respectively. Here ''U_id'' is selected fragment ID, while ''f'' is any fragment ID for which the corresponding score is > 0. What contact map, C<sub>NOE_B</sub> or C<sub>NOE_F</sub>, is shown on the graphs is indicated on the bar at the bottom of FG window. Clicking on this bar using mouse will switch from one contact map to another.</span></div><div>The contact map <span>C<sub>HNCA</sub> is shown on this graph implicitly as well by a color of graph bars.</span></div><div> For example, on the Figure the elements of contact map <span>C<sub>NOE_F</sub></span> <span>related to the fragment 21 are shown. Scores for the fragment 21 to be before the fragments 37, 20 and 17 in the protein sequence are shown in magenta, which means that these connections being derived from NOESY data are supported by HNCA spectra as well. At the same time, scores for the fragment 21 to be before the fragments 22 and 4 are shown in red meaning that theses connections are not supported by HNCA spectra.</span></div><div> <br></div><div> </div><div> <br></div> | ||
=== '''View>Assignment''' === | === '''View>Assignment''' === | ||
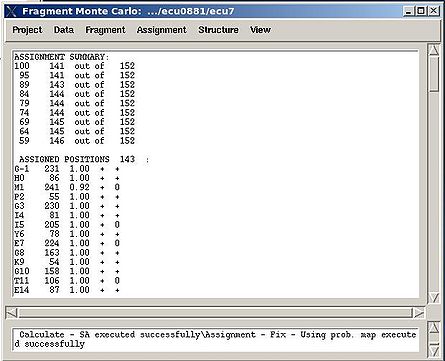
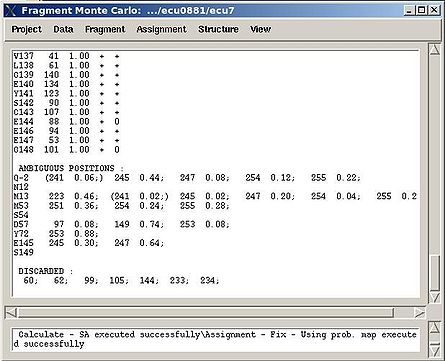
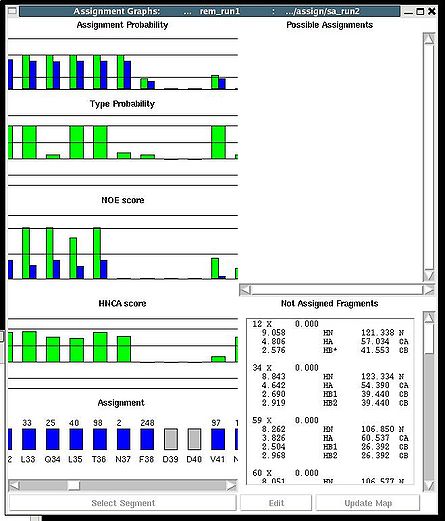
''':''': Display and analize assignment results | |||
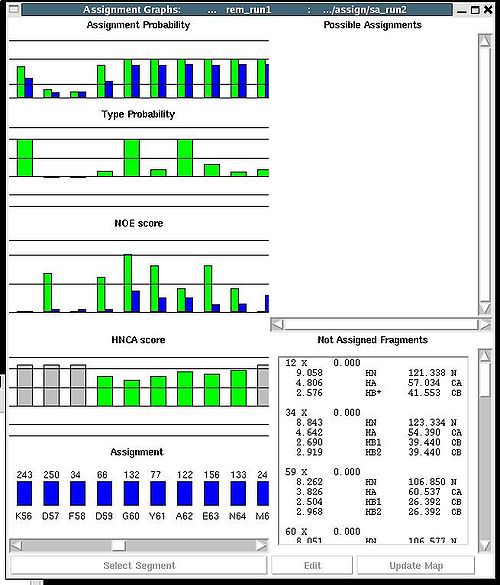
<div> </div><div>The user is asked to select a directory were assignment probabilities were calculated | <div> </div><div>The user is asked to select a directory were assignment probabilities were calculated (sa_run# or rem_run#). </div><div> New “Assignments Graphs” (AG) window pops up (see Figure 1, left). This window provides user with graphical means to manipulate PB fragment’s assignments without making changes of assignment status of PB fragments in memory. The current fragment’s assignment, taken from the memory, altogether with different scores associated with this assignment is shown when AG window is opened. The fragments that currently are not assigned are also shown in the bottom-right part of the AG window. User can modify the current assignment and to observe the resulting changes in the scores.</div><div>[[Image:Fmcgui fig2.22.jpg|thumb|left|445px]][[Image:Fmcgui 2.24a.jpg|thumb|right|445px]]</div><div></div><div></div><div> <br></div> | ||
<br> | <br> | ||
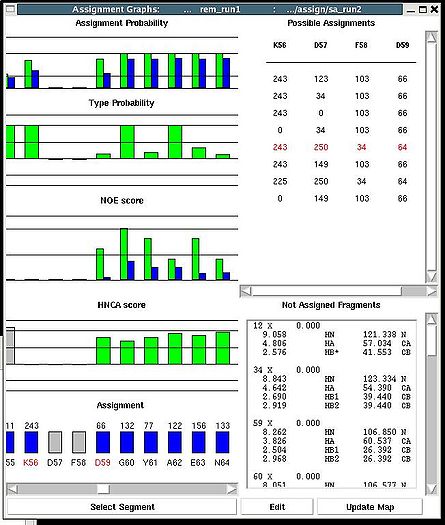
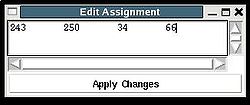
<div>Once a new assignment for the sequence segment is selected the current fragments assignment shown on the left part of AG window can be modified by pressing button “Update” (see Figure | <div><br> </div><div> A particular fragment’s assignment is displayed on the left part of the AG window. The graph at the bottom shows ID of fragments assigned to each sequence position, while four graphs on the top show different scores that correspond to this assignment, namely, HNCA score, NOE scores, typing and assignment probabilities that currently loaded in memory. The scroll bar at the bottom allows user to move along protein sequence. <br></div><div> <br></div><div>In order to modify the assignment user have first to select protein sequence segment of interest by clicking on IDs of two residues that correspond to the edges of the segment. The colour of these residues, for example K56 and D59, will change to red (see Figure 1,right). Then pressing on “Select Segment” bar at the bottom of AG window will result in possible assignment for the selected window to be shown on top-right corner of AG window. <div>[[Image:Fmcgui fig2.24.jpg|thumb|right|250px]]</div></div><div>The possible assignments for the selected sequence segment are taken form the selected directory shown on the title bar and correspond to sub-optimal fragment assignments sampled during SA or REM calculations. The new assignment for the sequence segment should be selected from the list of possible assignments using mouse. The color of the selected line will turn red (see Figure 2.23). In the case user want to consider an assignment that is not present in the list, he should select any assignment from the list ant modify it by pressing the button “Edit” at the bottom of the AG window. A new window “Edit Assignment” pops up, and user can modify the assignment by typing changes in this window see Figure 2). </div><div> <br></div><div><br> </div> <div>Once a new assignment for the sequence segment is selected the current fragments assignment shown on the left part of AG window can be modified by pressing button “Update” (see Figure 3)</div><div>[[Image:Fmcgui_fig2.25.jpg|thumb|center|500px]]</div><div> </div> | ||
Latest revision as of 23:33, 5 January 2010
Project Menu
Project>New
Project>Load
Project>Save
Poject>Quit
Data Menu
Data>Protein Sequence>Load
Data>Protein Sequence>Save as
Data>N15 NOESY>
Data>C13 NOESY>
Data>Arom NOESY>
Data>N15 HSQC>
Data>C13 HSQC>
Data>HNCA>
Data>HNCO>
Data>CBCACONHN>
Data>HBHACONH>
Data>Tolerances
There are six tolerances to set up:
NX - tolerance for matching resonances in N15 dimension
CX - tolerance for matching resonances in C13 dimension
HN - tolerance for matching resonances in HN direct dimension
HC - tolerance for matching resonances in HC direct dimension
Hi - tolerance for matching resonances in H indirect dimension
Ci - tolerance for matching resonances in C13 indirect dimension
Fragment>Load>assigned
Fragment>Load>PB fragments
Fragment>Save>PB fragments
- In the order of fragments index, that is in the order by which fragments are stored in memory;
- In the order of fragments user ID
- In the order of fragments assignment ID, A_id. In this case two files are saved. One file, with user specified name 'user_name', contains only fragments assigned to protein sequence positions, that is to positions with residue ID of >= 1. The second file, with the name 'user_name_na', contains all not assigned fragments (that is fragments with A_id = -99).
Fragment>Save>cyana
Fragment>Save>bmrb
Fragment>Save>talos
Fragment>Save>abacus
Fragment>Create>fawn
♦ loaded in memory referenced peak lists of CBCA(CO)HN, HBHA(CO)HN, N15HSQC, and HNCA spectra;
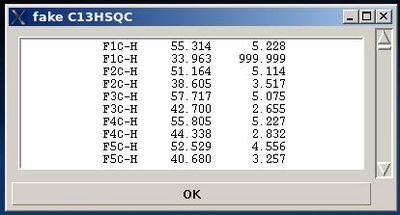
- On the first step, a fake C13HSQC peak list is created and shown in the popped up window “fake C13HSQC” .
2. On the second step, a number of bPB fragments corresponding to 20 different amino acid types are generated from user-identified spin-systems.
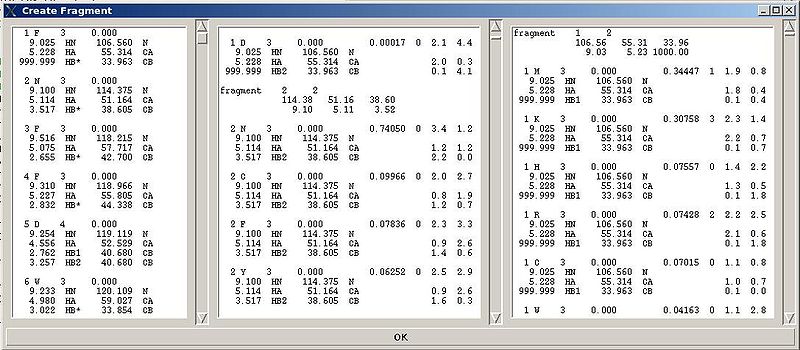
Each generated bPB fragment is evaluated by a score S(T) that measure how good the spin-system chemical shifts match corresponding statistical chemical shifts derived from BMRB database (see Figure 1.2). The bPB fragment with highest score is selected to form a list of bPB-fragments.
Fragment>Create>abacus
Figure 2.4
Following these warnings user can check and modify generated PB fragments in the left section of “Create Fragment’ window.
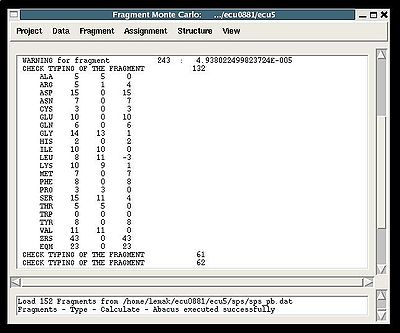
Fragment>Type>Calculate>
- the summary table that shows how many fragments of each AA-residue type are expected and how many fragments were actually recognized by the typing script
- warning messages that suggest user to check and possibly modify typing manually of some fragments manually (see command {Fragment>Type>fix})
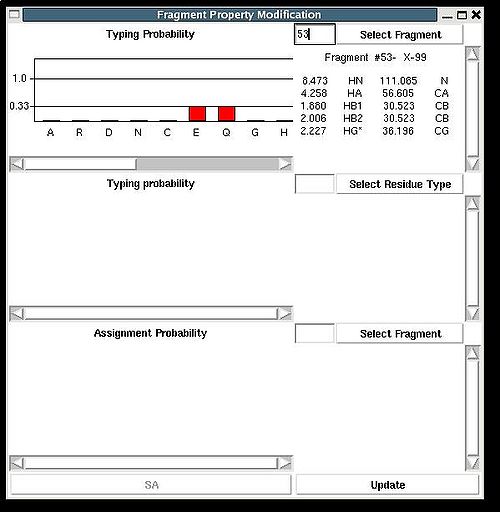
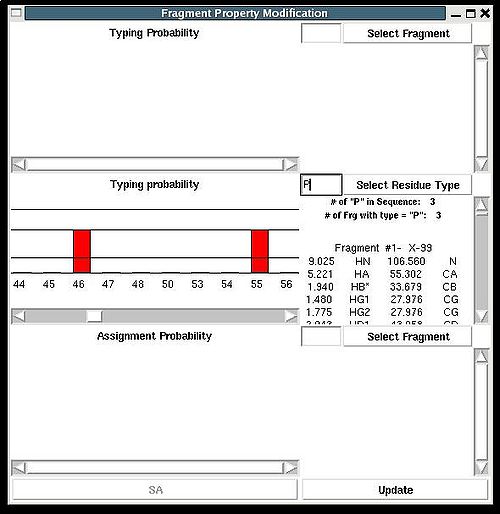
Fragment>Type>fix
Then the graph will show typing probabilities Tt1(f) <span />that correspond to the selected residue type t1 for all available fragments IDs f .
Fragment>Expected Peaks>
Prerequisites:
♦ protein sequence loaded in memory
♦ PB-fragments loaded in memory
Expected peak lists of the following spectra could be generated: N15-NOESY, C13-NOESY, H(C)CH-TOCSY, (H)CCH-TOCSY,
N15-HSQC, and C13-HSQC. Generated peak list is saved to the file on disk in SPARKY format.
Fragment>Modify assigned
♦ loaded in memory protein sequence
♦ loaded in memory PB-fragments
♦ loaded HNCO, CBCACONH, and HNCA peak lists.
♦ specified tolerances.
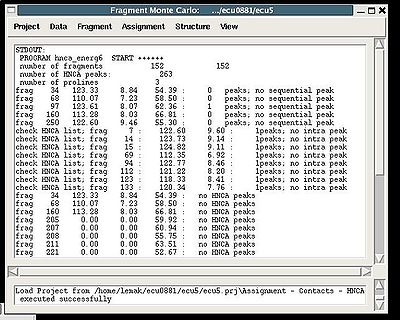
Assignment>Contacts>HNCA
:: Calculate fragments contact map CHNCA
Assignment>Contacts>NOE>fawn
Results:
Assignment>Contacts>NOE>abacus
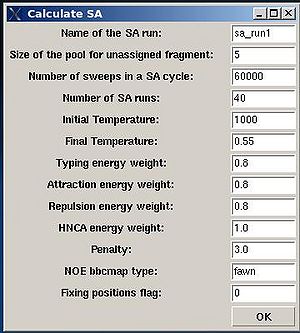
Assignment>Calculate Probabilities>SA
- “Name of the SA run”. Normally the name is sa_run#. A new directory under this name will be created within PROJECTNAME/assign directory. SA calculations will be curried out and the results will be stored in this directory.
- “Size of the pool for unassigned fragments”. The number of positions that are appended to the protein sequence and discarded (unassigned) fragments, if there are any, will be located there. It is safe to over-estimate this number. (If this number is under-estimated, this will force the mapping of spurious spin-systems onto protein sequence);
- “Number of SA trajectories”. The time needed for calculations is proportional to this number. On the other hand, having more SA trajectories the assignment probabilities could be calculated more accurately. In the case of good data, when all SA trajectories converge to assignments with the same energy, 10-15 trajectories should be enough. In the case of poor data, it is better to calculate 40-50 SA trajectories.
- “NOE bbcmap type”. User should specify which one NOE contact map, (abacus) or (fawn) should be used in the calculations;
- “Fixing position flag”. If the flag is set to 1, sequence position of all fragments which has assignment ID > -99 will be fixed during the simulation.
- “Final temperature”. Setting the optimal final temperature will provide all SA trajectories converge to optimal or sub-optimal assignments (the assignments that are in the vicinity of the global energy minimum). The optimal final temperature could be find by running one or a few SA runs with 3-4 trajectories and by analysing convergence of the trajectories from the report shown in the main FMCGUI window after each run (see Figure 2.10).
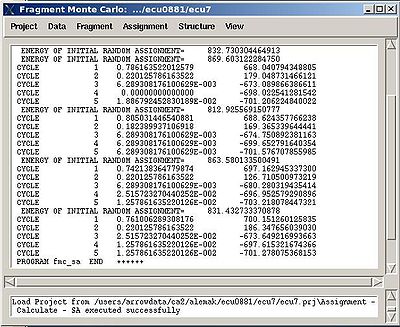
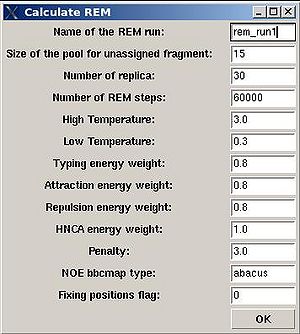
Assignment>Calculate Probabilities>REM
- “Name of the REM run”. Normally the name is rem_run#. A new directory under this name will be created within PROJECTNAME/assign directory. REM calculations will be curried out and the results will be stored in this directory.
- “Size of the pool for unassigned fragments”. The number of positions that are appended to the protein sequence and discarded (unassigned) fragments, if there are any, will be located there. It is safe to over-estimate this number. (If this number is under-estimated, this will force the mapping of spurious spin-systems onto protein sequence);
- “Number of REM steps”. The time needed for calculations is proportional to this number. On the other hand, with more REM steps more extensive sampling of assignment space wil be achieved, which in turn results in more accurate assignment probabilities. This number should be increased for large proteins.
- “NOE bbcmap type”. User should specify which one NOE contact map, (abacus) or (fawn) should be used in the calculations;
- “Fixing position flag”. If the flag is set to 1, sequence position of all fragments which has assignment ID > -99 will be fixed during the simulation.
- “Low temperature”. The optimal low temperature will provide extensive sampling of should sub-optimal assignments during REM simulation.
Assignment>Fix Assignment>Using Probability map
Results:
Assignment>Fix Assignment>Manually
♦ Assignment ID for selectedf PB fragments is specified
Assignment>Fix Assignment>Reset all
Assignment>Load Probabilities
Structure>Constraints>Talos>calculate
Structure>Constraints>Talos>load
Structure>Constraints>H-bonds>Specify
Structure>Constraints>H-Bonds>load
Structure>Calculate>Cyana
Structure>Calculate>ABCUS
Structure>RPF>RP
Results:
♦ summary report
- “RP directory name”. Normally the name is rp#. A new directory under this name will be created within PROJECTNAME/assign directory. The results of the RPFanalysis will be stored in this directory.
- “sequence gap”. Residue pairs separated by less than the value of sequence gap will be excluded from generating expected peak lists.
- “cutting distance for recall”. Distance threshold for evaluating matching of an experimental peak to a structural ensemble (Recall score)
- “cutting distance for precition”. Distance threshold for generating expected peak from structural ensemble (Precision score)
Structure>RPF>DP
- : Set up calculations of DP score with AutoStructure
Structure>Water refinement>calculate
- specify the name of directory for water refinement calculations, WATDIR;
- select a number of files with coordinates and constraints;
- indicate cisProline residues (if there are any)
- specify protonation state of HIS residues (which is double protonated by default).
Structure>Water refinement>summary
Structure>Add ZN ligands
Structure>RCI
:: Calculate Random Coil Index
View>Fragment
View>Assignment
:: Display and analize assignment results