SDS page gel: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| (6 intermediate revisions by 2 users not shown) | |||
| Line 11: | Line 11: | ||
==== I. Sample Preparation ==== | ==== I. Sample Preparation ==== | ||
#Add 5 μL 4X Reducing Buffer to 15μL protein solution. Vortex sample to mix. | #Add 5 μL 4X Reducing Buffer (RB) to 15μL protein solution. Vortex sample to mix. | ||
#Heat samples at 70 °C for 10 mins. | #Heat samples at 70 °C for 10 mins. | ||
#Spin samples. | #Spin samples. | ||
==== II. NuPage Gels ==== | ==== II. NuPage Gels ==== | ||
#Remove precast gels (4-12 % Bis-Tris gels from Invitrogen) from package. Cut open and dump out liquid in sink. Wash outside with MilliQ water. Pull off white stripe at bottom.<br> | |||
#Lower buffer core into cell and insert gel cassettes on both sides (make sure the shorter well side faces inward). Insert tension wedge and lock in place. (*If only running one gel, be sure to place buffer dam between the core and the tension wedge).<br> | |||
#Remove precast gels (4-12 % Bis-Tris gels from Invitrogen) from package. Cut open and dump out liquid in sink. Wash outside with MilliQ water. Pull off white stripe at bottom.<br> | #Fill the buffer core with 200 mL 1X RB. Make sure this section does NOT leak before adding liquid to the outside. Carefully remove the comb and pipette out any air bubbles. Then, fill outer part of case with 600 mL of RB.<br> | ||
#Lower buffer core into cell and insert gel cassettes on both sides (make sure the shorter well side faces inward). Insert tension wedge and lock in place. (*If only running one gel, be sure to place buffer dam between the core and the tension wedge).<br> | #Fill the wells with samples:<br> | ||
#Fill the buffer core with 200 mL 1X RB. Make sure this section does NOT leak before adding liquid to the outside. Carefully remove the comb and pipette out any air bubbles. Then, fill outer part of case with 600 mL of RB.<br> | #*Add 10 μL MW markers to well #1.<br> | ||
#Fill the wells with samples:<br> | #*Fill remaining 11 wells with 10 μL of prepared protein sample solution. | ||
#Add 10 μL MW markers to 1 | #*Fill each well slowly. Try to avoid bubbles in wells that may cause overflow into other wells<br> | ||
#Fill remaining 11 wells with 10 μL of prepared protein sample solution. | #*When working with 2 gel plates, make sure to label the outside of the container.<br> | ||
#When working with 2 gel plates, make sure to label the outside of the container.<br> | |||
#Match the + and -electrode ends (same colors) | #Match the + and -electrode ends (same colors) | ||
# | #*Turn on (be sure Range Select is OFF). Set Range to 200V/400mA, then hit Start. | ||
# | #*Run for 35 min. When done, then turn off.<br> | ||
#Gel stain: | #Gel stain: | ||
#*Take gel cassette out of Invitrogen gel box. | |||
#*Fill microwave safe glass container with MilliQ water enough as to immerse gel. | |||
#*Crack open gel cassette and place gel in microwave safe glass container. | |||
#*Microwave gel for two minutes or until water boils. | |||
#*Place container on shaker until cool. Then decant the water. Rinse one more time with MilliQ water. | |||
#*Add Denville Blue Protein Stain to gel container and immerse gel. | |||
#*Microwave gel for two minutes or until protein stain solution boils and place on shaker until cool. | |||
#*Decant gel stain into waste container, and rinse gel with MilliQ water. | |||
#*Add MilliQ water to immerse gel and microwave for two minutes and then shake until cool. | |||
#*Carefully place gel into plastic gel box filled with water. | |||
[[Image:Nupage gel.gif|left]] | |||
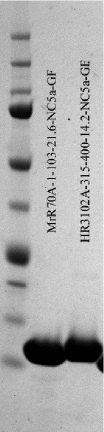
An example of a SDS-PAGE gel run with two protein samples is shown here. Lane#1: Marker, Lane#2: MrR70A-NC5, Lane#3: HR3102A-NC5. | |||
The marker used is [http://www.bio-rad.com Bio-Rad] catalog #161-6374 with molecular weights 10, 15, 20, 25, 37, 50, 75, 100, 150, 250 kDa from bottom to top. | |||
Latest revision as of 20:58, 3 February 2010
Rutger's University SDS Page Gel Protocol
Each NESG sample is run on an SDS page gel in order to confirm the monomer size and to look for the presence of SDS-resistant oligomers. These gels are also used to confirm sample purity for each of the labeled samples for NMR.
Invitrogen NuPage Gel Electrophoresis
I. Sample Preparation
- Add 5 μL 4X Reducing Buffer (RB) to 15μL protein solution. Vortex sample to mix.
- Heat samples at 70 °C for 10 mins.
- Spin samples.
II. NuPage Gels
- Remove precast gels (4-12 % Bis-Tris gels from Invitrogen) from package. Cut open and dump out liquid in sink. Wash outside with MilliQ water. Pull off white stripe at bottom.
- Lower buffer core into cell and insert gel cassettes on both sides (make sure the shorter well side faces inward). Insert tension wedge and lock in place. (*If only running one gel, be sure to place buffer dam between the core and the tension wedge).
- Fill the buffer core with 200 mL 1X RB. Make sure this section does NOT leak before adding liquid to the outside. Carefully remove the comb and pipette out any air bubbles. Then, fill outer part of case with 600 mL of RB.
- Fill the wells with samples:
- Add 10 μL MW markers to well #1.
- Fill remaining 11 wells with 10 μL of prepared protein sample solution.
- Fill each well slowly. Try to avoid bubbles in wells that may cause overflow into other wells
- When working with 2 gel plates, make sure to label the outside of the container.
- Add 10 μL MW markers to well #1.
- Match the + and -electrode ends (same colors)
- Turn on (be sure Range Select is OFF). Set Range to 200V/400mA, then hit Start.
- Run for 35 min. When done, then turn off.
- Gel stain:
- Take gel cassette out of Invitrogen gel box.
- Fill microwave safe glass container with MilliQ water enough as to immerse gel.
- Crack open gel cassette and place gel in microwave safe glass container.
- Microwave gel for two minutes or until water boils.
- Place container on shaker until cool. Then decant the water. Rinse one more time with MilliQ water.
- Add Denville Blue Protein Stain to gel container and immerse gel.
- Microwave gel for two minutes or until protein stain solution boils and place on shaker until cool.
- Decant gel stain into waste container, and rinse gel with MilliQ water.
- Add MilliQ water to immerse gel and microwave for two minutes and then shake until cool.
- Carefully place gel into plastic gel box filled with water.
An example of a SDS-PAGE gel run with two protein samples is shown here. Lane#1: Marker, Lane#2: MrR70A-NC5, Lane#3: HR3102A-NC5.
The marker used is Bio-Rad catalog #161-6374 with molecular weights 10, 15, 20, 25, 37, 50, 75, 100, 150, 250 kDa from bottom to top.