AutoStructure Theory: Difference between revisions
No edit summary |
No edit summary |
||
| (15 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
< | AutoStructure <ref>Huang, Y.J., Tejero, R., Powers, R., and Montelione, G.T., A topology-constrained distance network algorithm for protein structure determination from NOESY data. Proteins, 2006. 62(3): p. 587-603.</ref> is an automated NOESY assignment engine, which uses a distinct bottom-up topology-constrained approach for iterative NOE interpretation and structure determination. AutoStructure first builds an initial chain fold based on intraresidue and sequential NOESY data, together with characteristic NOE patterns of secondary structures, including helical medium-range NOE interactions and interstrand b-sheet NOE interactions, and unambiguous long-range NOE interactions, based on chemical shift matching and NOESY spectral symmetry considerations. NOESY cross peaks that cannot be uniquely assigned using these methods are not used in the initial structure calculations. Once initial structures are generated and validated, additional NOESY cross peaks are iteratively assigned using the intermediate 3D structures and contact maps, together with knowledge of high-order topology constraints of alpha-helix and beta-sheet packing geometries. This protocol, in principle, resembles the method that an expert would utilize in manually solving a protein structure by NMR. | ||
intraresidue and sequential NOESY data, together with characteristic NOE | |||
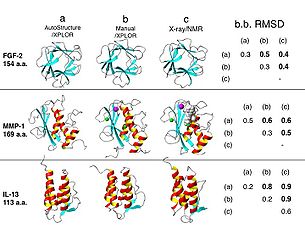
patterns of secondary structures, including helical medium-range NOE | The input data for AutoStructure are: (i) resonance assignment table, (ii) 2D, 3D, and/or 4D NOESY peak lists, (iii) list of scalar coupling, RDC and slow amide exchange data. AutoStructure generates distance constraint lists and utilizes the programs DYANA/CYANA, Xplor for 3D structure generation on a Linux-based computer cluster. Fig. 1 shows AutoStructure results for three different human protein NMR test data sets: FGF-2, IL-13 and MMP-1, ranging in size from 113 to 169 amino-acid residues. The mean coordinate differences between structures determined by AutoStructure and by manual analysis (0.5 to 0.8 Å for backbone atoms of ordered residues) demonstrate good accuracy of these automated methods. | ||
interactions and interstrand | |||
NOE interactions, based on chemical shift matching and NOESY spectral symmetry | [[Image:AS.jpg|left|307x229px|Ribbon diagrams of representative structures of FGF-2, MMP-1 and IL-13 proteins used for the validation of the AutoStructure process]] | ||
considerations. NOESY cross peaks that cannot be uniquely assigned using these | |||
methods are not used in the initial structure calculations. | |||
principle, resembles the method that an expert would utilize in manually | |||
solving a protein structure by NMR. | |||
table, (ii) 2D, 3D, and/or 4D NOESY peak lists, (iii) list of scalar | |||
coupling, RDC and slow amide exchange data. AutoStructure generates | |||
distance constraint lists and utilizes the programs DYANA/CYANA, Xplor | |||
generation on a Linux-based computer cluster. | |||
<br> | <br> | ||
{| cellspacing="1" cellpadding="1" border="1" style="width: 427px; height: 137px;" | |||
|- | |||
| Fig. 1. Ribbon diagrams of representative structures of FGF-2, MMP-1 and IL-13 proteins used for the validation of the AutoStructure process: (a) final structures from AutoStructure using XPLOR for stucture generation, (b) manual-analyzed structures deposited in PDB, analyzed using the same NMR data set, (c) structures determined by X-ray crystallography or third NMR group. Tabulated on the right are mean coordinate differences (Å) in secondary structure regions for backbone atoms between structures (a), (b) and (c). | |||
|} | |||
<br> | <br> | ||
<br> | |||
< | <br> | ||
AutoStructure's ‘bottom up’ strategy is quite different from the “top down” strategies used by the alternative programs CANDID and ARIA, which rely on “ambiguous constraints”. For NOESY spectra with poor signal-to-noise ratios, such automatically assigned ‘ambiguous constraint” sets may not include any true NOESY assignments, and result in small distortions of the protein structure which maybe avoided by the “bottom up” approach of AutoStructure. CANDID/CYANA also uses a ‘network anchoring” approach similar to, but less comprehensive than, the topology-constrained approach used by AutoStructure. For these reasons, some users may prefer to use both AutoStructure and CANDID/CYANA or ARIA in parallel to assess potential errors in automated NOESY cross peak assignments | |||
<br> <references /> | |||
Latest revision as of 21:00, 18 December 2009
AutoStructure [1] is an automated NOESY assignment engine, which uses a distinct bottom-up topology-constrained approach for iterative NOE interpretation and structure determination. AutoStructure first builds an initial chain fold based on intraresidue and sequential NOESY data, together with characteristic NOE patterns of secondary structures, including helical medium-range NOE interactions and interstrand b-sheet NOE interactions, and unambiguous long-range NOE interactions, based on chemical shift matching and NOESY spectral symmetry considerations. NOESY cross peaks that cannot be uniquely assigned using these methods are not used in the initial structure calculations. Once initial structures are generated and validated, additional NOESY cross peaks are iteratively assigned using the intermediate 3D structures and contact maps, together with knowledge of high-order topology constraints of alpha-helix and beta-sheet packing geometries. This protocol, in principle, resembles the method that an expert would utilize in manually solving a protein structure by NMR.
The input data for AutoStructure are: (i) resonance assignment table, (ii) 2D, 3D, and/or 4D NOESY peak lists, (iii) list of scalar coupling, RDC and slow amide exchange data. AutoStructure generates distance constraint lists and utilizes the programs DYANA/CYANA, Xplor for 3D structure generation on a Linux-based computer cluster. Fig. 1 shows AutoStructure results for three different human protein NMR test data sets: FGF-2, IL-13 and MMP-1, ranging in size from 113 to 169 amino-acid residues. The mean coordinate differences between structures determined by AutoStructure and by manual analysis (0.5 to 0.8 Å for backbone atoms of ordered residues) demonstrate good accuracy of these automated methods.
| Fig. 1. Ribbon diagrams of representative structures of FGF-2, MMP-1 and IL-13 proteins used for the validation of the AutoStructure process: (a) final structures from AutoStructure using XPLOR for stucture generation, (b) manual-analyzed structures deposited in PDB, analyzed using the same NMR data set, (c) structures determined by X-ray crystallography or third NMR group. Tabulated on the right are mean coordinate differences (Å) in secondary structure regions for backbone atoms between structures (a), (b) and (c). |
AutoStructure's ‘bottom up’ strategy is quite different from the “top down” strategies used by the alternative programs CANDID and ARIA, which rely on “ambiguous constraints”. For NOESY spectra with poor signal-to-noise ratios, such automatically assigned ‘ambiguous constraint” sets may not include any true NOESY assignments, and result in small distortions of the protein structure which maybe avoided by the “bottom up” approach of AutoStructure. CANDID/CYANA also uses a ‘network anchoring” approach similar to, but less comprehensive than, the topology-constrained approach used by AutoStructure. For these reasons, some users may prefer to use both AutoStructure and CANDID/CYANA or ARIA in parallel to assess potential errors in automated NOESY cross peak assignments
- ↑ Huang, Y.J., Tejero, R., Powers, R., and Montelione, G.T., A topology-constrained distance network algorithm for protein structure determination from NOESY data. Proteins, 2006. 62(3): p. 587-603.