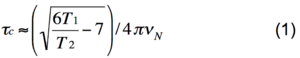Measuring 15N T1 and T2 relaxation times (Bruker): Difference between revisions
(Created page with '== '''Introduction''' == The rotational correlation time of a protein in solution is the time for a protein to rotate one radian. In the limit of slow molecular motion (τ<sub>c…') |
No edit summary |
||
| Line 1: | Line 1: | ||
== '''Introduction''' == | == '''Introduction''' == | ||
The rotational correlation time of a protein in solution is the time for a protein to rotate one radian. In the limit of slow molecular motion (τ<sub>c</sub> >> 0.5 ns), the correlation time of a protein is related to the ratio of the longitudinal (T<sub>1</sub>) and transverse (T<sub>2</sub>) <sup>15</sup>N relaxation times, and nuclear frequency (ν<sub>N</sub>) according to Eq. 1, which is derived from Eq. 8 in Kay et al. (Ref. 1) by considering only J(0) and J(ω) spectral densities and neglecting higher frequency terms. | The rotational correlation time of a protein in solution is the time for a protein to rotate one radian. In the limit of slow molecular motion (τ<sub>c</sub> >> 0.5 ns), the correlation time of a protein is related to the ratio of the longitudinal (T<sub>1</sub>) and transverse (T<sub>2</sub>) <sup>15</sup>N relaxation times, and nuclear frequency (ν<sub>N</sub>) according to Eq. 1, which is derived from Eq. 8 in Kay et al. (Ref. 1) by considering only J(0) and J(ω) spectral densities and neglecting higher frequency terms. [[Image:Tc eq1.png|center|300x60px]] | ||
| In practice, global <sup>15</sup>N T<sub>1</sub> and T<sub>2</sub> relaxation times for an unknown protein target can be obtained quickly (ca. 1 h) on a 1.7-mm microcyroprobe using 1D <sup>15</sup>N-edited relaxation experiments (Ref. 2), by fitting integrated signal in the backbone amide <sup>1</sup>H region of the spectrum as a function of delay time to an exponential decay. One then computes the correlation time using Eq. 1, and compares it to a standard curve of τ<sub>c</sub> vs. protein molecular weight (MW) obtained at the same temperature on a series of known monomeric proteins of varying size. As a general rule of thumb, the τ<sub>c</sub> of a monomeric protein in solution in nanoseconds is approximately 0.6 times its molecular weight in kiloDaltons. This approach is reliable up to MW ≈ 25 kDa, where accurate measurement of the diminishing <sup>15</sup>N T<sub>2</sub> becomes problematic. | ||
<br> | |||
== '''Methods''' == | |||
=== Acquisition of 1D <sup>15</sup>N T<sub>1</sub> and T<sub>2</sub> on Bruker Spectrometers === | |||
On Bruker spectrometers equipped with TopSpin 2.1 we use the [[Media:Hsqct1etf3gpsi3d.txt|hsqct1etf3gpsi3d]] and [[Media:Hsqct2etf3gpsi3d.txt|hsqct2etf3gpsi3d]] pulse sequences for the acquisition of <sup>15</sup>N T<sub>1</sub> and T<sub>2</sub> spectra (Ref. 1-3). The 1D version of these experiments are acquired as pseudo 2D spectra, where the <sup>1</sup>H<sup>N</sup> dimension is arrayed vs. delay time. We acquire <sup>1</sup>H-detected 1D <sup>15</sup>N relaxation spectra by using the following settings in the acquisition mode: | |||
*set the number of acquisition points in the <sup>15</sup>N dimension, td2 = 1 | |||
*set the number of points in the 3rd dimension equal to the number of delay times used in the experiment | |||
*set the acquisition mode in the 3rd dimension to QF | |||
*set NBL equal to the number of delay times used in the experiment; this is very important for the experiment to work properly | |||
*set the number of acquisition points in the <sup>15</sup>N dimension, td2 = 1 | *define a vdlist (T<sub>1</sub>) or vclist (T<sub>2</sub>) | ||
*set the number of points in the 3rd dimension equal to the number of delay times used in the experiment | |||
*set the acquisition mode in the 3rd dimension to QF | |||
*set NBL equal to the number of delay times used in the experiment; this is very important for the experiment to work properly | |||
*define a vdlist (T<sub>1</sub>) or vclist (T<sub>2</sub>) | |||
*set d1 to 3 - 5 s (T<sub>1</sub>) or 1.5 - 3 s (T<sub>2</sub>) for adequate relaxation during the experiment. | *set d1 to 3 - 5 s (T<sub>1</sub>) or 1.5 - 3 s (T<sub>2</sub>) for adequate relaxation during the experiment. | ||
Typical [[ | Typical [[Media:RelaxT1_122807.txt|vdlist]] and [[Media:Relaxt2_122807.txt|vclist]] files are attached.<br><br> | ||
Revision as of 19:48, 10 November 2009
Introduction
The rotational correlation time of a protein in solution is the time for a protein to rotate one radian. In the limit of slow molecular motion (τc >> 0.5 ns), the correlation time of a protein is related to the ratio of the longitudinal (T1) and transverse (T2) 15N relaxation times, and nuclear frequency (νN) according to Eq. 1, which is derived from Eq. 8 in Kay et al. (Ref. 1) by considering only J(0) and J(ω) spectral densities and neglecting higher frequency terms.
In practice, global 15N T1 and T2 relaxation times for an unknown protein target can be obtained quickly (ca. 1 h) on a 1.7-mm microcyroprobe using 1D 15N-edited relaxation experiments (Ref. 2), by fitting integrated signal in the backbone amide 1H region of the spectrum as a function of delay time to an exponential decay. One then computes the correlation time using Eq. 1, and compares it to a standard curve of τc vs. protein molecular weight (MW) obtained at the same temperature on a series of known monomeric proteins of varying size. As a general rule of thumb, the τc of a monomeric protein in solution in nanoseconds is approximately 0.6 times its molecular weight in kiloDaltons. This approach is reliable up to MW ≈ 25 kDa, where accurate measurement of the diminishing 15N T2 becomes problematic.
Methods
Acquisition of 1D 15N T1 and T2 on Bruker Spectrometers
On Bruker spectrometers equipped with TopSpin 2.1 we use the hsqct1etf3gpsi3d and hsqct2etf3gpsi3d pulse sequences for the acquisition of 15N T1 and T2 spectra (Ref. 1-3). The 1D version of these experiments are acquired as pseudo 2D spectra, where the 1HN dimension is arrayed vs. delay time. We acquire 1H-detected 1D 15N relaxation spectra by using the following settings in the acquisition mode:
- set the number of acquisition points in the 15N dimension, td2 = 1
- set the number of points in the 3rd dimension equal to the number of delay times used in the experiment
- set the acquisition mode in the 3rd dimension to QF
- set NBL equal to the number of delay times used in the experiment; this is very important for the experiment to work properly
- define a vdlist (T1) or vclist (T2)
- set d1 to 3 - 5 s (T1) or 1.5 - 3 s (T2) for adequate relaxation during the experiment.