Measuring 15N T1 and T2 relaxation times (Bruker): Difference between revisions
No edit summary |
m (moved Measuring 15N T1 and T2 Relaxation Times on Bruker Instruments to Measuring 15N T1 and T2 relaxation times (Bruker)) |
(No difference)
| |
Latest revision as of 21:36, 16 December 2009
Methods
Acquisition of 1D 15N T1 and T2 on Bruker Spectrometers
On Bruker spectrometers equipped with TopSpin 2.1 we use the hsqct1etf3gpsi3d and hsqct2etf3gpsi3d pulse sequences for the acquisition of 15N T1 and T2 spectra (Ref. 1-3). The 1D version of these experiments are acquired as pseudo 2D spectra, where the 1HN dimension is arrayed vs. delay time. We acquire 1H-detected 1D 15N relaxation spectra by using the following settings in the acquisition mode:
- set the number of acquisition points in the 15N dimension, td2 = 1
- set the number of points in the 3rd dimension equal to the number of delay times used in the experiment
- set the acquisition mode in the 3rd dimension to QF
- set NBL equal to the number of delay times used in the experiment; this is very important for the experiment to work properly
- define a vdlist (T1) or vclist (T2)
- set d1 to 3 - 5 s (T1) or 1.5 - 3 s (T2) for adequate relaxation during the experiment.
Typical vdlist and vclist files are attached.
At Rutger's University, spectra are obtained in about 1hr on a 600 MHz Bruker TCI 1.7-mm MicroCyroprobe. This probe provides S/N that is about one order of magnitute higher than a conventional 5-mm probe.
Processing 1D 15N T1 and T2 on Bruker Spectrometers
- To process the pseudo 2D 15N relaxation date, type:
xf2
You will be prompted for a new processed data number under you experiment name.
- Next, extract the first row (into ~TEMP file) and basline correct
- Return to the processed pseudo2D apply the baseline correction using the command
bas
- Relaxation data is analyzed using the t1guide package (below) within TopSpin.
Analyzing 1D 15N T1 and T2 Data Using t1guide
For detailed information on the t1guide routine, consult the help documentation within Topspin 2.1 under Help -> Manuals -> General User Manual.
- In Topsin 2.1, launch t1guide by typing:
t1guide
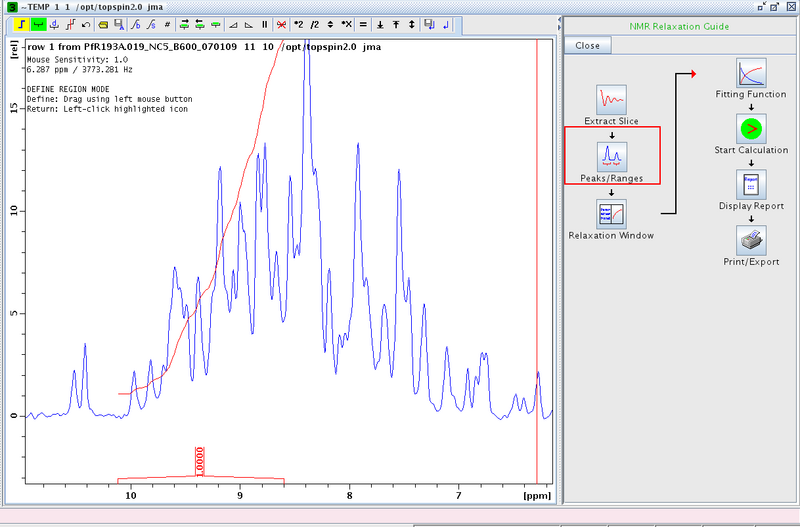
- Extract the first slice of the spectrum using the Extract Slice option and expand on the amide region.
- Proceed to Peaks/Ranges and perform a manual integration (Figure 2). We typically integrate between δ ≈ 10 to 8.5 ppm. Save the integration region to disk (the diskette/A symbol in the tool bar).
Figure 1: Manual Integration of Amide Region in First Slice
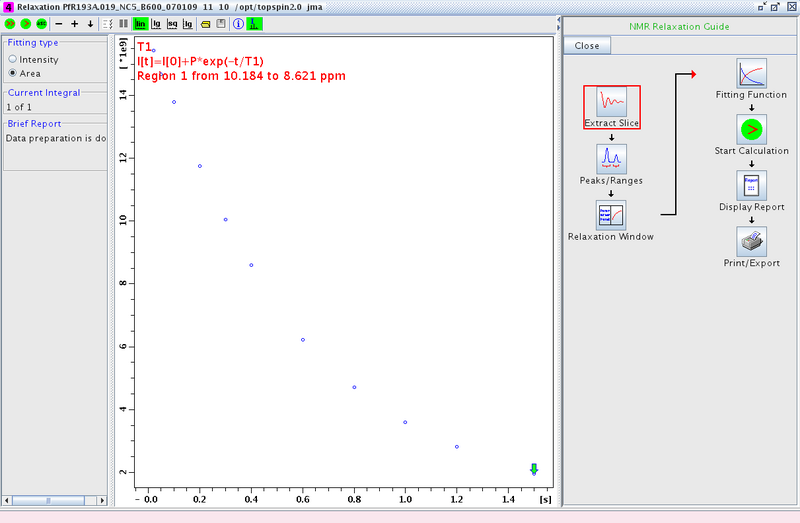
- Proceed to the Relaxation Window. A plot of integral area vs. delay time will be displayed (Figure 2). Any points that are outliers can be deleted at this point using the mouse.
Figure 2: Relaxation Window
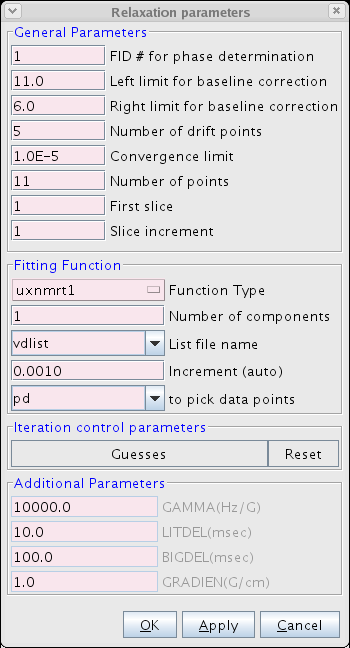
- Proceed to the Fitting Function (Figure 3). Here the user inputs various parameters including the number of points and type of function used to fit the data. We typically use the uxnmrt1 function and we prefer to use the integral area. Be sure to select the correct vdlist or vclist used in the experiment.
Figure 3: The Fitting Function
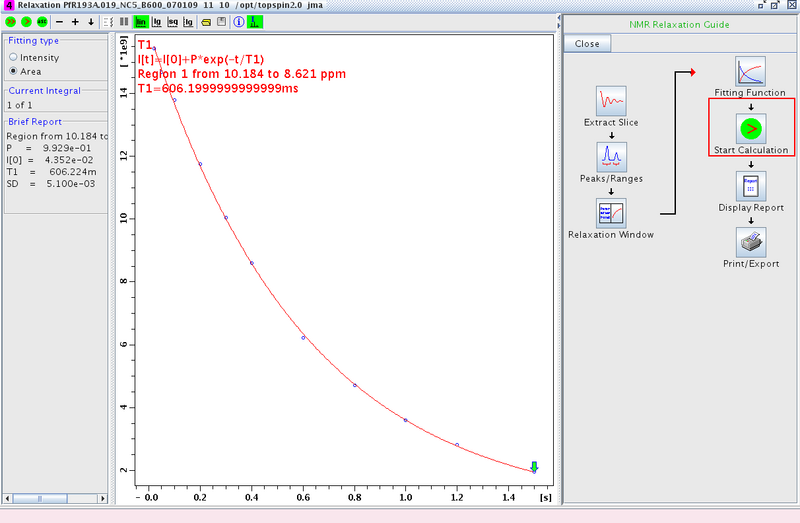
- Proceed to Start Calculation and fit the data (Figure 4)
Figure 4: Fitting the Data
- Finally, the user can also display/print the report and print the plot.
Compare the New τc to a Standard Plot
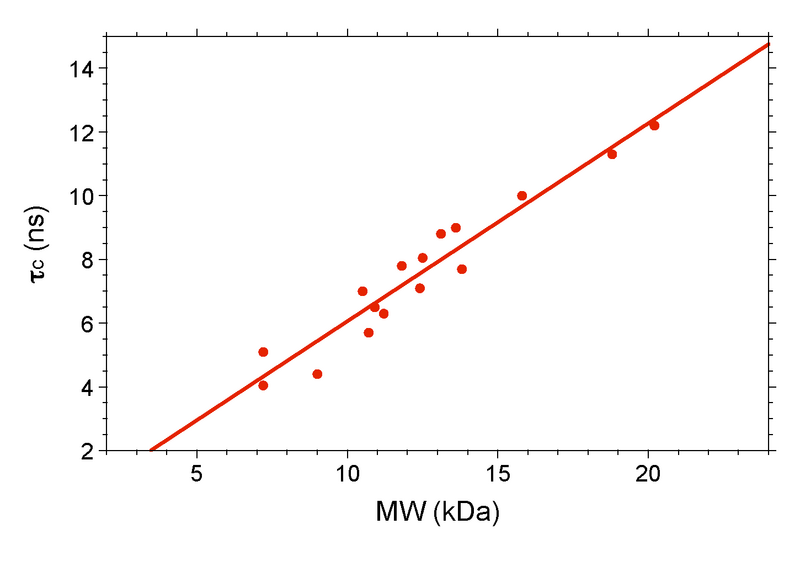
The final step in the process is to compare your τc to a standard plot of τc (ns) vs. MW (kDa) for known monomers (Figure 5). Temperature is an extremely important variable here, and it is recommended that all data be acquired at the same temperature, preferably on the same instrument. At Rutgers we have a standard curve obtained at 298 K on our Bruker 600. If your target is significantly above the curve, then it is not a monomer.
Figure 5: Plot of τc vs. MW for Various NESG Monomers
References
1. Kay, L.E., Torchia, D.A. and Bax, A. (1989) Backbone dynamics of proteins as studied by 15N inverse detected heteronuclear NMR spectroscopy: Application to staphylococcal nuclease. Biochemistry 28, 8972-8979.
2. Farrow, N.A., Muhandiram, R., Singer, A.U., Pascal, S.M., Kay, C.M., Gish, G., Shoelson, S.E., Pawson, T., Forman-Kay, J.D. and Kay, L.E. (1994) Backbone dynamics of a free and phosphopeptide-complexed Src homology 2 domain studied by 15N NMR relaxation. Biochemistry 33, 5984-6003.
3. Barbato, G., Ikura, M., Kay, L.E., Pastor, R.W. and Bax, A. (1992) Backbone dyanamics of calmodulin studied by 15N relaxation using inverse detected two-dimensional NMR spectroscopy. Biochemistry 31, 5269-5278.
-- JimAramini - 10 Nov 2009